SilverFox
Flashaholic
"What is a good rate to charge my NiMh cells?" and "Is this charger good?" are frequent questions that come up around here. Slow charging has been around forever, and it is used in determining cycle life. However, there is a provision in the testing standard for "Accelerated Test Procedures," when testing for cycle life. The accelerated procedure involves charging and discharging at 1C. The goal is to achieve more than 500 cycles. The accelerated test procedures have a footnote that states that 1C charging should be done for 1 hour, or with appropriate charge termination, as recommended by the manufacturer.
The next step is to review what the battery manufacturers have to say about "appropriate charge termination."
All of the battery manufacturers recommend charging at either 0.1C for 16 hours, or in the range of 0.5 – 1.0C with proper charge termination. They list peak voltage, change in temperature with respect to time, and total temperature rise as the preferred termination signals, but also recognize that –dV can also be used.
NiCd charging started with simple timed chargers. From there, they realized that NiCd chemistry was fairly robust, and most of the time cells were being charged from more, or less, empty. They then advanced to 3 or 5 hour charging. Realizing that there was a voltage drop as the cell reached full charge, the fast chargers were ushered in. Now we had 0.5 to 2 hour chargers available. The chip manufacturers jumped in and gave us an abundance of charging chips that allowed us to select charging rates and utilized –dV to terminate the charge.
When NiMh cells became available, the charger manufacturers realized that they could use the same NiCd chargers to charge NiMh cells. However, further study indicated that the overcharging involved using the –dV values for NiCd cells when charging NiMh cells resulted in around a 20% loss of cycle life. This was a direct result of overcharging and heat.
The charger manufacturers realized that –dV termination was easy to implement and fairly reliable, so they approached the chip manufacturers about modifying their existing chips to accommodate NiMh charging. The result was increasing the "dead time" where the charger would not accept a fluctuation in voltage as a end of charge termination signal, and reducing the value of the –dV from 10 – 15 mV to 3 – 5 mV. They also come up with charging in pairs, thinking that the combined voltage drop from two cells would be easier to detect.
With a few exceptions, most of the quick or smart NiMh chargers available today utilize –dV charge termination. If you are checking out a charger, you should probably assume that it utilizes –dV termination, unless it states something different.
When picking out a charger for NiMh cells, the first thing you need to know is how does it determine charge termination. Once you know that, you can then check to see if the charging rate of the charger is suitable to produce a strong end of charge signal. In the case of chargers that utilize –dV termination, the suitable charge rate is in the range of 0.5 – 1.0C.
The next thing to look at is the trickle charge rate at the end of the charge. If this rate is too high, and you leave your cells on the charger, you will cook your cells and greatly reduce your cycle life. The optimum is to have the charger trickle for awhile, then shut off. However, some people become over concerned with the self discharge rate of NiMh cells and want a charger that keeps the cell at a full state of charge through trickle charging. In this case, you want a very low trickle charge rate. You can refer to the various battery manufacturers for their definition of what a suitable trickle charge rate is. I don't recommend leaving cells on the charger trickle charging, but if that is what you think you need to do, I believe the newer Maha chargers trickle charge at a low enough rate that the cells may actually self discharge a little while still on trickle charge. That seems like a suitable rate to me…
So much for the introduction, now let's take a look at charging rates.
The Schulze charger has a long term formatting setting that they label 0.1C charging. There is no time limit or charge termination when using this setting. The charger continues to pulse charge until you manually stop it. The duty cycle is 25 seconds at 400 mA followed by 75 seconds at 0 mA. I use this setting for forming cells and battery packs, and balancing battery packs.
I have noticed, from time to time, that there is a voltage drop during this slow charge. It doesn't always show up, but with healthy cells and a balanced pack, it usually does.
Let's take a look at one of my 4400 mAh NiMh B90 prototype packs with less than 100 charge/discharge cycles on it. This pack was charged and balanced, then partially discharged for this study. Peak voltage was reached at around 110 minutes into the charge. It took 6300 seconds (105 minutes) to develop a –dV of 2 mV per cell.
Here is the graph:
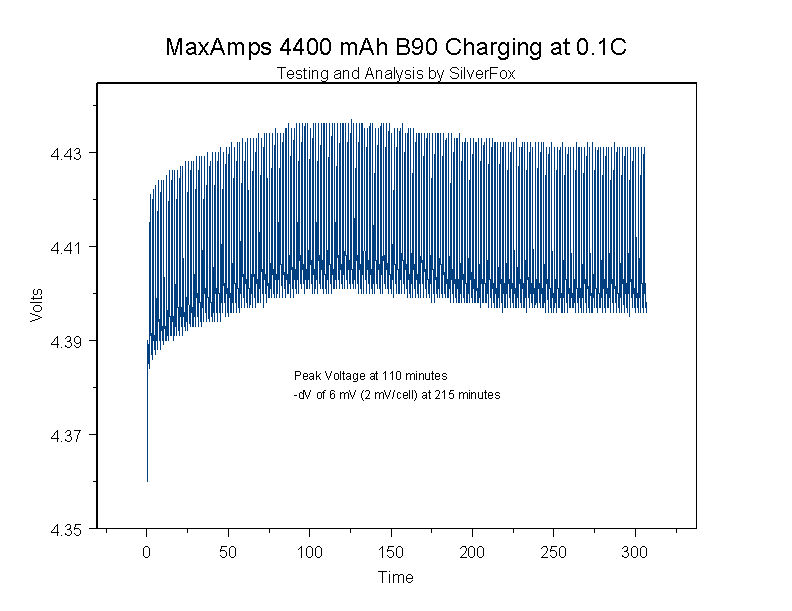
You may be curious why I listed the time to –dV drop in seconds. Checking out the specifications on the charging chips available from the various chip manufacturers, I have observed that when they are looking for a –dV value, they use a time ranging from 100 – 300 seconds. If I was using one of these chips hoping to terminate this charge, I would miss the signal because of the long time (6300 seconds) it took to develop the signal.
The result is a missed termination.
Fortunately, at the 0.1C charge rate, there is little consequence with a missed termination. However, if you increase the charge rate, you can run into problems.
A Swedish study looked at the affects of a 30% overcharge. It found that charging at 0.3C with a 30% overcharge yielded around 225 charge/discharge cycles before the cell capacity dropped to below 80% of its initial capacity. Charging at 1.0C with proper termination (actually they were using a –dV of 10 mV which I think is high. They would have obtained better results using a –dV of 2 mV, but this study was done a few years ago) yielded around 500 cycles before the capacity dropped to below 80%. The study goes on to illustrate that overcharging at 1.0C is more detrimental than overcharging at 0.3C, but the point is well made that overcharging, even at lower charge rates, is bad for the health of the cell.
OK, let's throw some numbers out… If you have a 2000 mAh cell, a 0.3C charge rate would be 600 mA. If your charger misses the end of charge termination, it will continue to charge until the safety timer shuts off. If your safety timer is set to 3000 - 3300 mAh (BC-900) or 4000 mAh (C-9000), or 8050 mAh (Vanson BC1HU), you will end up with an overcharged cell. A 30% overcharge on a 2000 mAh cell is roughly 2600 mAh.
I have some aged AAA Moden 850 mAh cells. These cells were revived from near death due to low voltage due to improper long term storage. They are not the most vibrant cells, but they do a good job at lower current draws. I use them in my Peak Matterhorn that I EDC on my key chain.
Here is a graph while charging at 0.25 amps (roughly 0.3C):
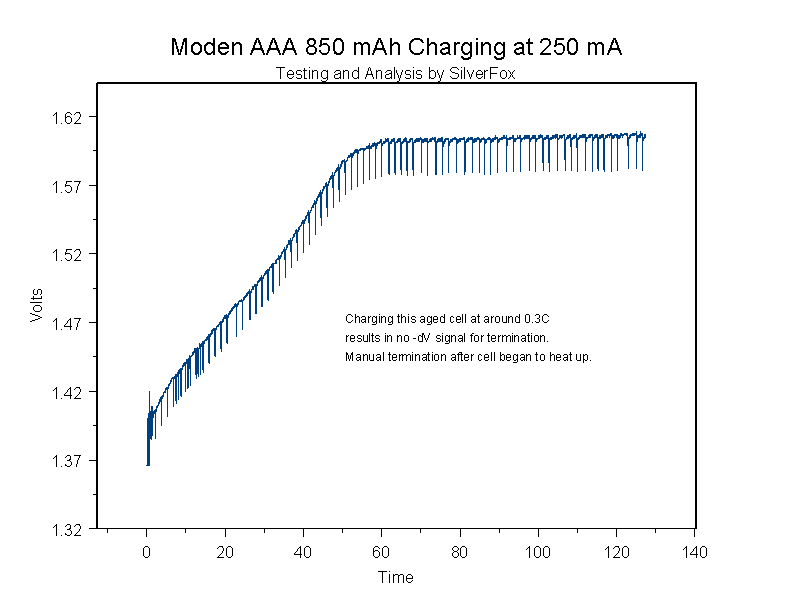
As you can see, there was no –dV signal. I terminated the charge because the cell started to warm up more than "normal." If I repeatedly charged this cell at this rate on a charger that utilized –dV termination, I would end up with reduced cycle life due to overcharging.
Here is the same cell charging at 0.5 amps:
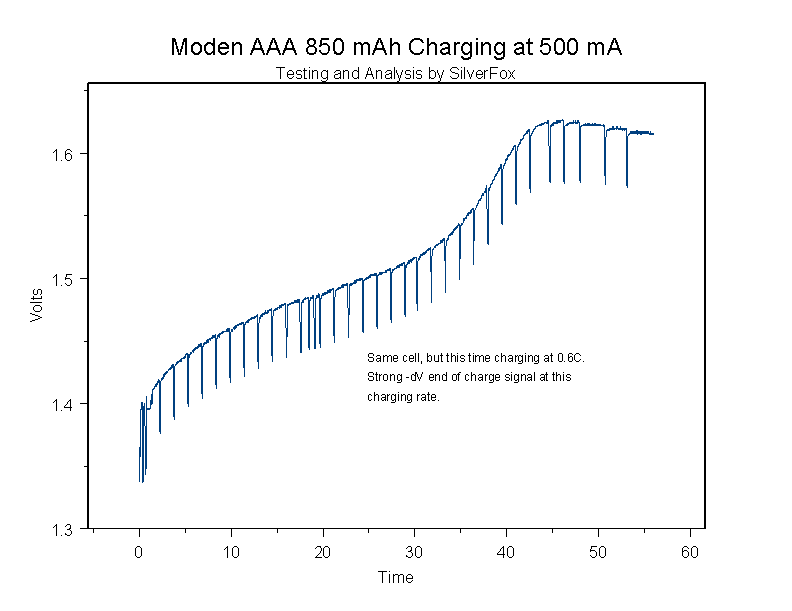
At this rate there is a strong end of charge signal, and the problem with overcharging is eliminated.
Getting back to the original questions… What is the best rate to charge NiMh cells at? It depends on the method your charger uses to terminate the charge. If your charger utilizes –dV termination, you will get a more reliable signal if you charge in the range of 0.5 – 1.0C.
Now, if your charger utilizes peak voltage termination, things change. If you look at the graphs, you can see that the voltage rises to a point, then does not rise further. If you terminate the charge when the voltage stops rising, this can be observed regardless of the charge rate. You will notice that it is present in the 0.1C charge of the 4400 mAh battery, in the 0.3C charge of the 850 mAh cell, and also in the 0.6C charge of the 850 mAh cell.
When using peak voltage termination, you now need to look beyond termination to determine the best charging rate for your cells. Slow charging produces large crystals, and large crystals produce voltage depression. Once again, ultra slow charging may not give you the best performance. Now we are in an area that is application dependent. I have often heard that you should charge at about the same rate that your application uses the cells. There may be a lot of truth in this.
It is interesting to observe the habits of the RC people. Their NiMh packs used in their cars, trucks, airplanes, and helicopters are often charged at 2 – 3C, but the battery packs in their transmitters and controllers are usually charged at 0.1C for 14 – 16 hours. While their vehicle battery packs are only prime for around 20 – 50 cycles, their transmitter packs seem to last a long time.
The questions remains, Why does Sanyo in the Eneloop site seem to contradict the recommendations in their main site? Perhaps the Eneloop chargers have changed from –dV termination to peak voltage termination. I think some testing may be in order…
Tom
The next step is to review what the battery manufacturers have to say about "appropriate charge termination."
All of the battery manufacturers recommend charging at either 0.1C for 16 hours, or in the range of 0.5 – 1.0C with proper charge termination. They list peak voltage, change in temperature with respect to time, and total temperature rise as the preferred termination signals, but also recognize that –dV can also be used.
NiCd charging started with simple timed chargers. From there, they realized that NiCd chemistry was fairly robust, and most of the time cells were being charged from more, or less, empty. They then advanced to 3 or 5 hour charging. Realizing that there was a voltage drop as the cell reached full charge, the fast chargers were ushered in. Now we had 0.5 to 2 hour chargers available. The chip manufacturers jumped in and gave us an abundance of charging chips that allowed us to select charging rates and utilized –dV to terminate the charge.
When NiMh cells became available, the charger manufacturers realized that they could use the same NiCd chargers to charge NiMh cells. However, further study indicated that the overcharging involved using the –dV values for NiCd cells when charging NiMh cells resulted in around a 20% loss of cycle life. This was a direct result of overcharging and heat.
The charger manufacturers realized that –dV termination was easy to implement and fairly reliable, so they approached the chip manufacturers about modifying their existing chips to accommodate NiMh charging. The result was increasing the "dead time" where the charger would not accept a fluctuation in voltage as a end of charge termination signal, and reducing the value of the –dV from 10 – 15 mV to 3 – 5 mV. They also come up with charging in pairs, thinking that the combined voltage drop from two cells would be easier to detect.
With a few exceptions, most of the quick or smart NiMh chargers available today utilize –dV charge termination. If you are checking out a charger, you should probably assume that it utilizes –dV termination, unless it states something different.
When picking out a charger for NiMh cells, the first thing you need to know is how does it determine charge termination. Once you know that, you can then check to see if the charging rate of the charger is suitable to produce a strong end of charge signal. In the case of chargers that utilize –dV termination, the suitable charge rate is in the range of 0.5 – 1.0C.
The next thing to look at is the trickle charge rate at the end of the charge. If this rate is too high, and you leave your cells on the charger, you will cook your cells and greatly reduce your cycle life. The optimum is to have the charger trickle for awhile, then shut off. However, some people become over concerned with the self discharge rate of NiMh cells and want a charger that keeps the cell at a full state of charge through trickle charging. In this case, you want a very low trickle charge rate. You can refer to the various battery manufacturers for their definition of what a suitable trickle charge rate is. I don't recommend leaving cells on the charger trickle charging, but if that is what you think you need to do, I believe the newer Maha chargers trickle charge at a low enough rate that the cells may actually self discharge a little while still on trickle charge. That seems like a suitable rate to me…
So much for the introduction, now let's take a look at charging rates.
The Schulze charger has a long term formatting setting that they label 0.1C charging. There is no time limit or charge termination when using this setting. The charger continues to pulse charge until you manually stop it. The duty cycle is 25 seconds at 400 mA followed by 75 seconds at 0 mA. I use this setting for forming cells and battery packs, and balancing battery packs.
I have noticed, from time to time, that there is a voltage drop during this slow charge. It doesn't always show up, but with healthy cells and a balanced pack, it usually does.
Let's take a look at one of my 4400 mAh NiMh B90 prototype packs with less than 100 charge/discharge cycles on it. This pack was charged and balanced, then partially discharged for this study. Peak voltage was reached at around 110 minutes into the charge. It took 6300 seconds (105 minutes) to develop a –dV of 2 mV per cell.
Here is the graph:

You may be curious why I listed the time to –dV drop in seconds. Checking out the specifications on the charging chips available from the various chip manufacturers, I have observed that when they are looking for a –dV value, they use a time ranging from 100 – 300 seconds. If I was using one of these chips hoping to terminate this charge, I would miss the signal because of the long time (6300 seconds) it took to develop the signal.
The result is a missed termination.
Fortunately, at the 0.1C charge rate, there is little consequence with a missed termination. However, if you increase the charge rate, you can run into problems.
A Swedish study looked at the affects of a 30% overcharge. It found that charging at 0.3C with a 30% overcharge yielded around 225 charge/discharge cycles before the cell capacity dropped to below 80% of its initial capacity. Charging at 1.0C with proper termination (actually they were using a –dV of 10 mV which I think is high. They would have obtained better results using a –dV of 2 mV, but this study was done a few years ago) yielded around 500 cycles before the capacity dropped to below 80%. The study goes on to illustrate that overcharging at 1.0C is more detrimental than overcharging at 0.3C, but the point is well made that overcharging, even at lower charge rates, is bad for the health of the cell.
OK, let's throw some numbers out… If you have a 2000 mAh cell, a 0.3C charge rate would be 600 mA. If your charger misses the end of charge termination, it will continue to charge until the safety timer shuts off. If your safety timer is set to 3000 - 3300 mAh (BC-900) or 4000 mAh (C-9000), or 8050 mAh (Vanson BC1HU), you will end up with an overcharged cell. A 30% overcharge on a 2000 mAh cell is roughly 2600 mAh.
I have some aged AAA Moden 850 mAh cells. These cells were revived from near death due to low voltage due to improper long term storage. They are not the most vibrant cells, but they do a good job at lower current draws. I use them in my Peak Matterhorn that I EDC on my key chain.
Here is a graph while charging at 0.25 amps (roughly 0.3C):

As you can see, there was no –dV signal. I terminated the charge because the cell started to warm up more than "normal." If I repeatedly charged this cell at this rate on a charger that utilized –dV termination, I would end up with reduced cycle life due to overcharging.
Here is the same cell charging at 0.5 amps:

At this rate there is a strong end of charge signal, and the problem with overcharging is eliminated.
Getting back to the original questions… What is the best rate to charge NiMh cells at? It depends on the method your charger uses to terminate the charge. If your charger utilizes –dV termination, you will get a more reliable signal if you charge in the range of 0.5 – 1.0C.
Now, if your charger utilizes peak voltage termination, things change. If you look at the graphs, you can see that the voltage rises to a point, then does not rise further. If you terminate the charge when the voltage stops rising, this can be observed regardless of the charge rate. You will notice that it is present in the 0.1C charge of the 4400 mAh battery, in the 0.3C charge of the 850 mAh cell, and also in the 0.6C charge of the 850 mAh cell.
When using peak voltage termination, you now need to look beyond termination to determine the best charging rate for your cells. Slow charging produces large crystals, and large crystals produce voltage depression. Once again, ultra slow charging may not give you the best performance. Now we are in an area that is application dependent. I have often heard that you should charge at about the same rate that your application uses the cells. There may be a lot of truth in this.
It is interesting to observe the habits of the RC people. Their NiMh packs used in their cars, trucks, airplanes, and helicopters are often charged at 2 – 3C, but the battery packs in their transmitters and controllers are usually charged at 0.1C for 14 – 16 hours. While their vehicle battery packs are only prime for around 20 – 50 cycles, their transmitter packs seem to last a long time.
The questions remains, Why does Sanyo in the Eneloop site seem to contradict the recommendations in their main site? Perhaps the Eneloop chargers have changed from –dV termination to peak voltage termination. I think some testing may be in order…
Tom

