I just bought a 2600mah lithium polymer battery power bank.
Its specs say 5V-800 ma input
and 5V 1000 ma output.
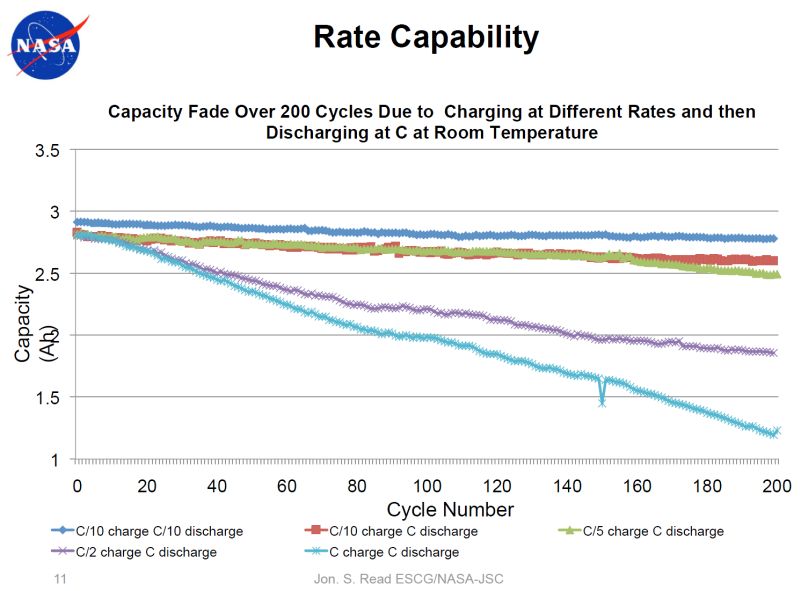
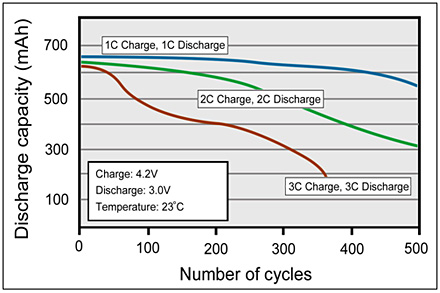
That seems strange to me, I would think that input and out put should be at least the same, and that it should be able to take much more input, at 1c.
3.7v/5v*2.6amp= 1.924 amp or 1924 ma.
I charged it with a 1 amp wall wart, and it didn't even get warm.
I have two USB ports in my car, one is 1 amp, the other 2.1 amps.
Thoughts?
Its specs say 5V-800 ma input
and 5V 1000 ma output.
That seems strange to me, I would think that input and out put should be at least the same, and that it should be able to take much more input, at 1c.
3.7v/5v*2.6amp= 1.924 amp or 1924 ma.
I charged it with a 1 amp wall wart, and it didn't even get warm.
I have two USB ports in my car, one is 1 amp, the other 2.1 amps.
Thoughts?