StorminMatt
Flashlight Enthusiast
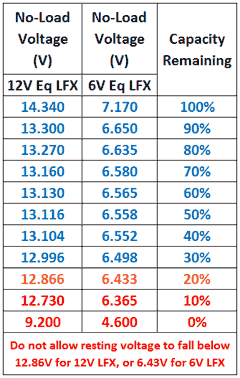
not true .. state of charge can be determined easily by a simple voltage reading. for single cell that takes a precision volt meter which the average consumer doesn't have. but for four cell in series like for 12v Motorcycle LiFePO4 batteries. it's easy to determine state of charge with a VOM.
here's a state of charge chart for 12v LiFePO4
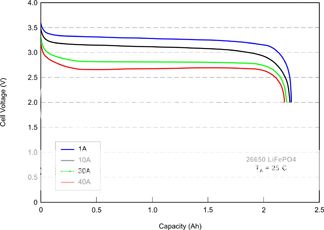
As far as determining the state of charge based on voltage, you can see here that there's only a .2V difference between 80% and 20% state of charge with 6V (or around half a volt for 12V). This is FAR, FAR, FAR less than you would see with, say, LiMn, LiCo, or lead acid. And it is well within the range of error you would get using even a very accurate DVM due to such factors as a little oxidation on the terminals or leads. With LiFePO4, a better way to measure capacity would be to know how much current you have been drawing for how long.
Anyway, speaking of the use of LiFePO4, it's also finding use for off grid solar power. Compared to lead acid, it not only has a more stable voltage. It also lasts longer and is less maintenance intensive. And it takes up FAR less space (and weighs less) for a given capacity. There is also no worry about losing a battery that never gets fully charged (say, due to extended cloudy/rainy weather in the short days of winter) from sulfation. LiFePO4 is more expensive than flooded lead acid. But it is actually quite cost competitive with AGM batteries.
Last edited: