How about more words to consider (member RCinFLA post#12)
This topic has been discussed many times here and I'm well aware that standard practice is that you should not attempt to charge below 32°F but I found the previous discussions lacking in rigor and specifics. Actual tests are difficult to do without access to electron microscopes and lots of...

diysolarforum.com
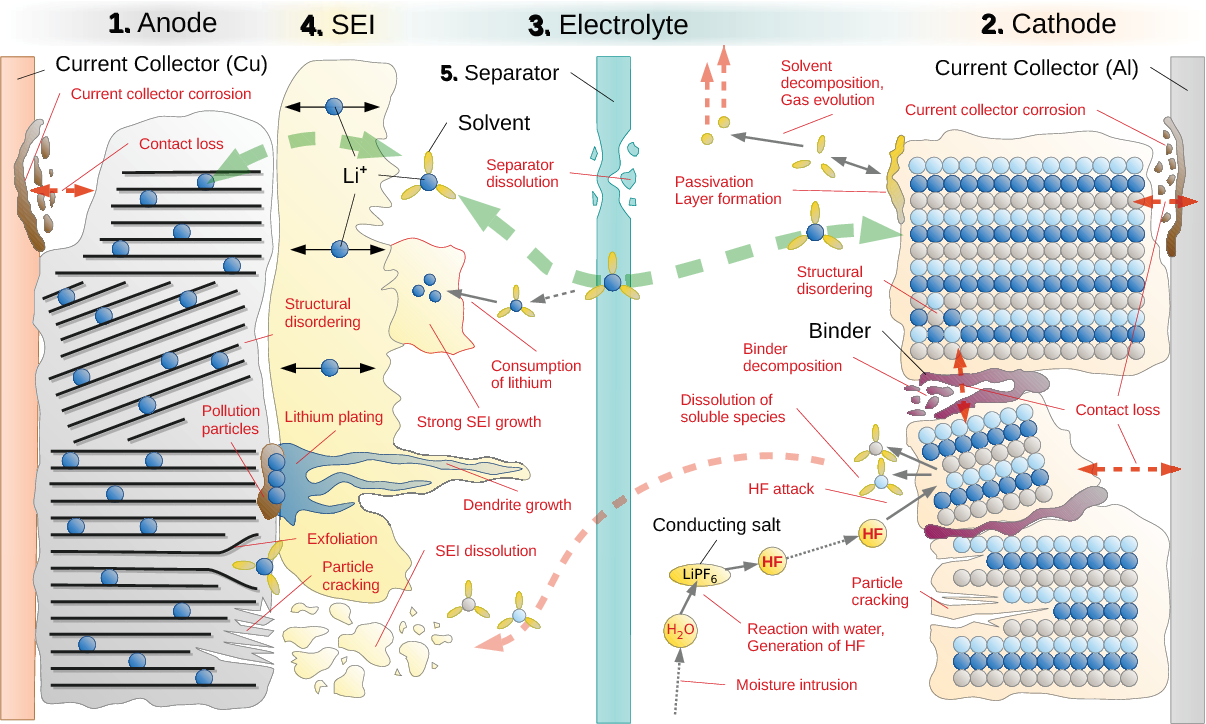
Most all lithium-ion batteries operate in pretty much the same way. Positive cathode supplies the lithium and graphite negative anode stores the lithium-ions in the charged state. Electrolyte provides the lithium-ion relay exchange conveyor belt via the dissolved lithium salts in the electrolyte hydrocarbon solvent.
Common to all lithium-ion batteries is you do not want electrons from outside terminals to meet lithium-ions within the cell. It is the job of positive and negative electrodes to make this charge exchange without allowing electrons and lithium-ions to chemically copulate. When they do, and it does to some small extent, damage to cell is done. Throwing too many lithium-ions at negative anode too quickly with high cell current during charging will allow more forbidden interaction between electrons and lithium-ions. This is cold temp charging issue.
Each manufacture has some minor variant 'special sauce' added to electrolyte and 'pixy dust' added to electrodes. These provide targeted improvements for a particular parameter (usually at the degradation of another parameter). For example, an electrolyte additive may improve electrolyte lithium-ion mobility at low temperatures. The latest 'pixy dust' trend is to salt a bit of silicon dust to negative graphite electrode to bump up AH capacity of cell (and improperly call it a solid-state battery), or pre-lithiated salts to negative graphite electrode to reduce the manufacturing process of initial charge forming that builds the protective Solid Electrolyte Interface (SEI) layer and reduces the lithium consumed from cathode during this process which reduces yielded sellable cell capacity.
Cell damage and aging falls broadly into two categories of electrode damage and electrolyte damage. Charging at cold temp is in the negative electrode damage category. Overcharging is in the electrolyte damage category. Over-discharging eats at the copper and aluminum foil electrode current collector interface contaminating the electrodes. This is just a few examples of a very long list of possible ways to have cell damage.
One special mention is accelerated damage to SEI layer due to excessive charge and discharge currents. This eats up free lithium on subsequent recharging to rebuild damaged SEI protection. Fully charging bloats up negative electrode graphite by 11-13% in volume, stuffing it with lithium-ions resulting in some cracking of SEI protective layer grown around the graphite. The SEI layer helps keep lithium-ions separate from electrons in negative graphite electrode.
High cell current also requires high overpotential to drive the demand for lithium-ion migration. The greater overpotential within layers of the cell encourages many other damaging parasitic chemical reactions. Each layer of cell has a safe voltage potential gradient/temp range. Too much or too little overall cell voltage, too much cell current, or too high cell temperature may drive a given layer outside its safe operating range making that layer vulnerable to damaging parasitic chemical reactions.
The holy grail is a solid-state negative graphite anode replacement that allows negative electrode to hold more lithium-ions for given volume. Silicon can store five times more lithium-ions within the silicon lattice compared to graphite, but silicon eats so many lithium-ions the silicon bursts destroying the silicon structure. Result is very poor cycle life of less than 200 cycles. Solid state electrolyte is another big effort to accomplish.