Guys,
I need to qualify myself as no artist what so ever beyond having some creative ideas but no technical skills to get them down by hand. (I have found more satisfaction and some rudimentary success in PhotoShop where I can take images I have captured with a camera and then manipulate them). That said, I think and have commented in the past, that a good artist could do some amazing things with titanium as a canvas and altering the surface finish and oxide film as means of creating an image. The resulting image is not only dependent on a light source but also its location relative to the observer. The image changes as the light source or observer move, relative to the image and the other. As a crude example, in the image below what appears as shaddow in the mountains (hope you can figure out which are the montains

) in the photo taken go from shadow to highlight and the highlighted areas go to shadow if you change your orientation to the Ti plate or move the light source.
The dark blue ocean with light colored wave lines goes to a shiny light blue ocean (polished surface) with darker wave lines (diamond burr etch) as the light orientation changes in the image below of the jewler's loop. Three rubbies were set in the Ti plate I made:
Heavy wear in contact and abrasion can damage a Ti canvas but beyond that, weather and light will not change it at all. I can't imagine a more permanent canvas or means of holding and retaining a graphic over the long haul.

of course the image should be one worthy of long term existence!
My wife and I made Ti earings many years ago and most were formed and cut thin sheets of Ti which were then polished, bead blasted engraved and masked and anodized in steps. I found you could get some really cool and subtle textures and colors using jeweler's polishing and grinding bits as well as tiny wire wheels and buffing wheels and points in conjunction with the anodizing. You can also subject the piece to a light dusting of bead blasting with blasting pens that are the scale of the small airburshes.
There are the classic mediums of scrimshaw, cloisene, and engraving that artisans practiced over the centuries and I can't help but imagine that if the technologies at the time provided titanium and and the means of anodizing it that some of these artisans wouldn't have embraced it as well. Perhaps someday, it will become a field in jewelery or forms of functional art where some real craftsmen and artists apply their magic! :thumbsup:
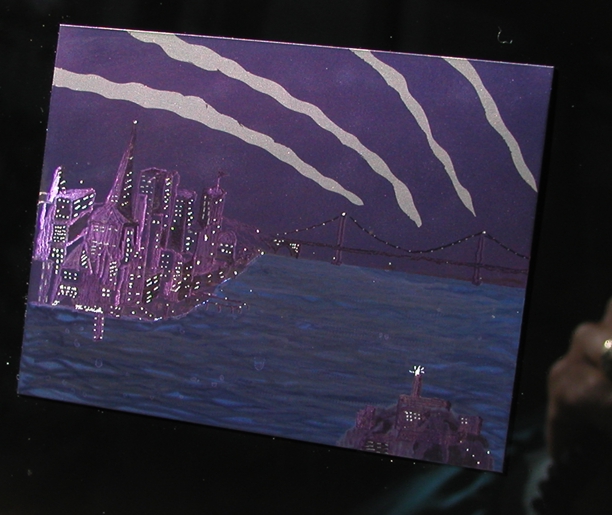
In the real crude depiction of the SF city front with golden gate in the background and Alacratraz in the bay, all of the little "lights" are the result of a tiny diamond burr that cut through the anodize. As you move the light source or your viewing angle from left to right, these lights blink on or off and the sky and water go from relative light to dark:
If you can consider these examples on par with a two year old's crayon drawings but then imagine the same medium in the hands of a master.....






 of course the image should be one worthy of long term existence!
of course the image should be one worthy of long term existence!