Disciple
Enlightened
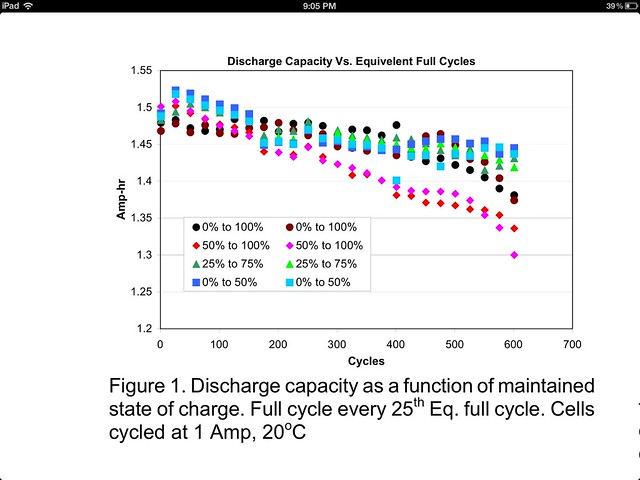
I recently made the jump into lithium ion powered lights using 18650 cells. I knew in advance that these cells have a finite lifespan (OK, what doesn't) but I don't think I gave it as much thought as I should have before purchasing. I only have a few flashlights  and I intend to keep my cells charged and in the lights as they are of little use to me otherwise. I am learning that Li-ion cells may age faster than I expected under these circumstances. The article How to Prolong Lithium-based Batteries from BatteryUniversity.com gives as a typical example a loss of 20% capacity after one year stored fully charged at 25°C. I do not know how much charge is lost the following year but it sounds like cells stored in this fashion will only have a useful lifespan of 2-3 years, or less if the aging accelerates.
and I intend to keep my cells charged and in the lights as they are of little use to me otherwise. I am learning that Li-ion cells may age faster than I expected under these circumstances. The article How to Prolong Lithium-based Batteries from BatteryUniversity.com gives as a typical example a loss of 20% capacity after one year stored fully charged at 25°C. I do not know how much charge is lost the following year but it sounds like cells stored in this fashion will only have a useful lifespan of 2-3 years, or less if the aging accelerates.
How much difference it would make to store cells charged to perhaps 85-90% capacity versus 100%? (That site only shows 40% and 100%.)
What would the lifespan of Samsung or Sanyo 4.35v cells charged only on a 4.2v charger be? (I know that initial capacity would be derated by this.)
 and I intend to keep my cells charged and in the lights as they are of little use to me otherwise. I am learning that Li-ion cells may age faster than I expected under these circumstances. The article How to Prolong Lithium-based Batteries from BatteryUniversity.com gives as a typical example a loss of 20% capacity after one year stored fully charged at 25°C. I do not know how much charge is lost the following year but it sounds like cells stored in this fashion will only have a useful lifespan of 2-3 years, or less if the aging accelerates.
and I intend to keep my cells charged and in the lights as they are of little use to me otherwise. I am learning that Li-ion cells may age faster than I expected under these circumstances. The article How to Prolong Lithium-based Batteries from BatteryUniversity.com gives as a typical example a loss of 20% capacity after one year stored fully charged at 25°C. I do not know how much charge is lost the following year but it sounds like cells stored in this fashion will only have a useful lifespan of 2-3 years, or less if the aging accelerates.How much difference it would make to store cells charged to perhaps 85-90% capacity versus 100%? (That site only shows 40% and 100%.)
What would the lifespan of Samsung or Sanyo 4.35v cells charged only on a 4.2v charger be? (I know that initial capacity would be derated by this.)
Last edited: