Li-ion beginner primer
12/27/11: I have explained the abbreviations and battery chemistries a bit at the end of the LI ION BATTERY CHEMISTRY AND SAFETY section. I added some words about using a DMM at the end of the recommendations section.
12/26/11: Added A BIT MORE DETAIL ON CHARGING, after the charging section
Hi folks, on other forums I read, I often notice a lack of info on Li Ion batteries. Most disconcerting, people have experience with alkaline and Nimh batteries, and assume Li Ion care and feeding is as simple, and the consequences of a mistake similarly (usually) small. I wrote a quick beginner primer, that may actually be useful here too, though folks here tend to be a lot more knowledgeable. In the meantime, I'm asking for feedback on this: is it factually correct? Are there important details I left out? Is it useful? Some of the emphasis in the article has been lost -- I was able to underline important points, but that underlining has disappeared in this forum (I'll change to bolding later today).
I'm going to try to make this really short, which means I'll drastically simplify and generalize. If anyone things I've oversimplified to point that the information looks incorrect, let me know and we'll discuss changing it right here in the primer. If you just want to add detail, we should let those stay as follow-ups. The reader should know that I've simplified a lot, you should be checking places like battery university and candlepowerforums for more detail; I mostly want to jumpstart you into a place where you're vaguely safe.
WHY WRITE A PRIMER?
I'll tell you first, I'm a bit conservative when it comes to things like safety. For all the positives of Li Ion batteries, the downside is that the consequences of making a mistake can be serious -- explode in your light or burn down your house serious. On the other hand, let's keep some perspective: many people use these batteries in hobbies like RC, and homes aren't burning down left and right. That said, I don't want *you* to be the statistic. I look at Li Ion batteries like a firearm: understand and follow a few simple rules, and there's no reason to fear them. But these are not the no-brainer technology that alkalines are.
I'm going to discuss the very basics of batteries and chargers. I'm going to use some examples of specific products that I feel violate the rules. And I'm going to try to give you enough info to evaluate that claim on your own. I will make specific recommendations for beginners at the end.
WHY GO WITH LI ION?
• If you use your light a lot, rechargeables will be much cheaper to run in the long run, and easier on the environment
• Modern lights are increasingly running at higher performance with Li Ion rechargeables. For example, the current XML based V10R is running 150 lumens on a CR123 primary, but over 400 lumens on a RCR123 or 16340 (both terms for a li ion rechargeable).
• Even though lithium primaries have higher capacity than lithium ion rechargeables, when I walk out the door with my rechargeable, I know my light is at full charge. With primaries, I'm never sure, since I'm not going to replace a primary that seems to be running well just because I've used it for the past week, and I can't just top it up.
LI ION BATTERY CHEMISTRY AND SAFETY
There are multiple battery chemistries for Li Ion batteries. I am going to almost solely discuss LiCo, but there are other chemistries popular in lights such as IMR and Lifepo4. I will contrast with IMR occasionally.
The thing about LiCo batteries is that the chemistry is not safe. By not safe, I mean, conditions can occur where the battery goes into thermal runaway, at which point it can vent violently or explode. A safer battery chemistry might cause the battery to shut down under these conditions, but because LiCo batteries go into runaway, I am only going to be discussing
protected batteries from here on out: those batteries with built-in protection including a protection circuit and other safeguards that shut the battery down and prevent other conditions that can cause problems. However, I'll note that I never rely 100% of mechanical protection, these are inexpensive parts that sometimes aren't perfect. Having a safety doesn't mean you can point a loaded gun at someone

IMRs, by contrast, use a safer battery chemistry, and so don't have protection circuits. But IMRs have lower overall capacity than protected LiCos. Still, as we'll see, IMRs have characteristics that can let them outperform LiCos under some circumstances.
A quick summary of the most popular chemistries:
LiCo (LiCoO2) aka ICR batteries:
• Are the batteries usually being discussed when someone mentions something like "protected AW 14500" without any other qualifiers
• 4.2V max charge, 3.7V nominal voltage
• Not a safe chemistry, so can be purchased with a protection circuit that guards against overcharging and over-discharging
• Higher energy density, but lower charge and discharge rates, than the other batteries in this list
• Are the batteries I use in lights that can handle 4.2V and do not demand a higher current than what LiCo can deliver
• Chemical composition is lithium cobalt oxide
IMR (LiMn204) batteries:
• 4.2V max charge, 3.7V nominal charge
• Safe chemistry, so is not available with a protection circuit. But that means the consumer must guard against overcharge and overdischarge themselves.
• Slightly lower energy density than LiCo, but can sustain much higher discharge and charge rates. I use these in lights that can handle 4.2V batteries but require a higher discharge rate than LiCo can handle, like some of the newer demanding XM-L lights
• Chemical composition is lithium manganese oxide
LiFePO4 aka IFR batteries:
• 3.6V max charge, 3.2V nominal voltage
• Safe chemistry, so is not available with a protection circuit. But that means the consumer must guard against overcharge and overdischarge themselves.
• Lower energy density than LiCo, but can sustain higher discharge and charge rates.
• Chemical composition is lithium iron phosphate
CHARGING
These batteries use a CC/CV charging algorithm, where in the first phase of charging, an initial constant current is used. When the battery reaches 4.2V, that voltage is kept constant, until the current dwindles to a percentage of the initial current ... and when that happens, the charge terminates. You may be as shocked as I was to learn that the vast majority of production chargers do NOT conform to the above well-known and manufacturer-approved specs. We'll talk about a few chargers that do, later. One thing we do want to do:
make sure we use a charger that's been shown to use the correct algorithm. But we don't want to completely depend on correct operation: as a rule, put your charger on a relatively fire resistant surface, stay with it while it's charging, and take your batteries off the charger when the light turns green. Technically, we've improved our safety by using a battery with overcharge protection and a charger that uses the proper algorithm; but if those two things go bad (and let's face it, with your luck, they might

), bad things can happen.
The other thing to watch out for is that a li ion that's been discharged too low is
not safe to recharge or use, in the light-your-house-on-fire sense. Again, we're talking about batteries with protection circuits that should protect against that, but those protection circuits cost a few cents and can go bad. Do not make it a habit of letting the protection circuit trip to tell you when to recharge. There's very little penalty for topping off Li Ion rechargeables, which is a nice feature of the technology, it means you can top off often. I suggest you do so. If the protection circuit fails to trip, you may be recharging from an unsafe voltage.
The other feature of recharging: you really don't want to recharge at over 1C for smaller LiCo batteries and .7C for larger ones –
check your battery's specs to see the safe charging rate for your battery. C stands for the capacity of the battery. For example, a protected RCR123 currently is usually rated at 750 mAh. That means you don't want to use a charger that uses a current of greater than 750 mA, and really, you should stick with .8*750mA=600mA. Note that IMR batteries have different charging specs, with rates nearly reaching 3C.
A few examples now: the highly rated Pila IBC charger has a charging current of 600mA. That means you're good with batteries as small as a protected RCR123. But you certainly should not charge a 10440 (350 mAh) or smaller on this charger. For those of you who followed the now-recalled Jetbeam charger, you may remember me being critical in those threads. That's because Jetbeam claimed 10440 support for this charger, even though the charging current for a single battery could be as high as 1000 mAh. That's nearly 3C, and way outside manufacturer safety recommendations!!! So, I hope you now know how to tell if a charger's charging current is safe for a particular Li Ion battery: look at the capacity written on the battery, and stick to the battery manufacturer's recommendation; .7C will usually be safe, when in doubt.
For reference, below is a chart of a CC/CV charge. The interesting points:
• Current starts out constant, with voltage rising
• When voltage hits 4.2V, it gets held constant, while current diminishes
• Current reduces until it hits a cut-off threshold (50mA, in this case), at which point the charge terminates (current should be zero or close to it, at the cutoff).
Many production chargers miss the mark on a number of points, in particular, the final termination. This could lead to dangerous conditions,
A BIT MORE DETAIL ON CHARGING
Below is a graph of a charger that correctly follows a CC/CV algorithm, courtesy of HKJ of CPF. I include it so you can see what a charging cycle should look like. Again, many chargers do not follow the algorithm properly. Among the common problems: at the end of the cycle, the charger must terminate, but many chargers keep applying what is essentially a trickle charge, which is arguably a dangerous mistake, and a good reason to choose a charger which has been reviewed and proven to follow a reasonable approximation of CC/CV, and to follow good charging practices.
Below, what you see in the first portion of the graph is the CC (constant current) stage. In this case, the current is kept constant at 1.0A while the voltage rises. When the voltage hits 4.2V, the CV (constant voltage) stage starts, where 4.2V is applied and the current naturally falls. When the current matches the termination current, you see the charger terminate at the vertical yellow line – we know proper termination has been achieved because current drops to 0 abruptly at that spot.
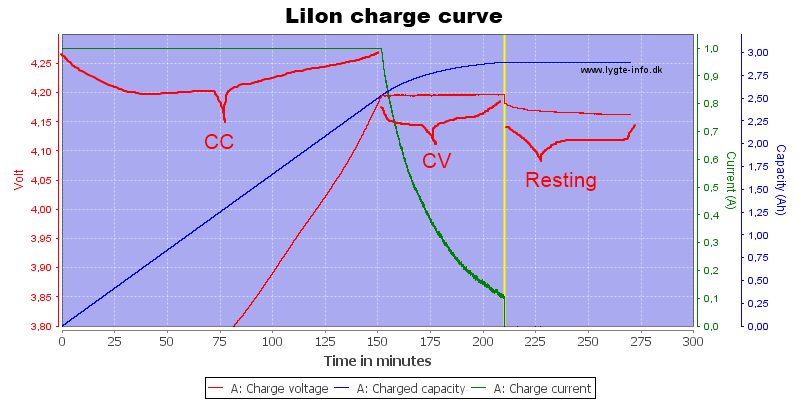
Another good practice is to have some way to measure the voltage of your Li Ion batteries, to make sure they're coming off the charger with safe voltages, that you haven't discharged to an unsafe voltage, etc. The LiCo batteries we've been discussing have been spec'ed to charge to 4.2V when fully charged, plus or minus .05V. Note that battery voltage will settle down a few hundredths of a volt, in the couple of hours after it comes of the charger; you can see that happening in the graph above, when the voltage represented by the red line starts sagging a bit after the yellow line indicating charge termination. If your battery is older, or you're using a spacer, you might see lower voltages, even below 4.15V. Using an inexpensive digital multimeter (DMM), or the voltmeter that's integrated into your Cottonpickers charger, you can check these voltages. I particularly do not want to see batteries coming off the charger higher than spec; if that happens, it's important to figure out why, and resolve the issue immediately. Below 1.5V, these batteries start forming shunts that can internally short the battery, leading to fire or explosion; if a battery gets down that low, I retire it.
USING (DISCHARGING) LI ION BATTERIES
LiCo batteries want to be discharged at no more than 2C (read the above section if you don't remember what "2C" means). For a 750 mAh protected RCR123, that means no more discharge than 1500 mA. IMR batteries can handle up to 8C. That means for a 16340 IMR at 550 mAh, no more than 4400 mA (4.4A). By knowing the current demand of a flashlight, you can tell how a battery will run.
More real-life examples. There are a couple of XML powered lights that run on a single CR123A or RCR123A/16340 to put out 400+ lumens. For example, Jetbeam and the Sunwayman V10R XML put out well over 400 lumens, and to reach that output, they are pulling well over 2A (2000 mA) from the battery. We know from experimenting, IMR batteries can outperform LiCo batteries in these lights, even though IMRs have lower capacity. Can you tell why, based on the discussion above? These lights are pulling way more than the manufacturer-recommended 2C for LiCo batteries, and those batteries sag quickly under the load. IMRs, which can handle the load, provide higher output for longer -- and the current requirements can be safely met by IMRs. I run an IMR in my v10r, and since IMRs do not have an overdischarge protection, I just take care to charge it often.
HOW DO I KNOW THE SPECS ABOVE?
You'll need to research some of the specs above yourself. I get the battery's manufacturer recommendations right from them. For example, AW posts the charge and discharge ratings on his batteries right on his forsale page on cpfmarketplace. I read reviews of chargers and lights to determine things like whether a charger is following the correct algorithm, what its initial charging current is, what a particular light's current draw on high is, etc. I learned most of what I know from the battery section on CPF, a good place for more advanced topics. I strongly suggest you be self-sufficient in determing safe ranges for your batteries and chargers – as I outlined above, there are flashlight and charger manufacturers making recommendations that are contrary to the recommendations from the battery manufacturers. I typically take the battery manufacturer's recommendations as primary, and will not use a battery in a light or charger whose specs are in contrast with the battery manufacturer's safe ranges.
RECOMMENDATIONS FOR BEGINNER SETUPS
Li Ion technology is not the place to cheap out. Buy quality from the beginning for both safety and top performance. Here are the recommendations I make to my friends who are just starting:
Buy your protected LiCo or IMR batteries from these manufacturers, who have proven to have very high quality high-performing batteries:
• AW
• Redilast
• Callie's Kustoms
Buy your charger from these manufacturers, who have been shown in reviews to use proper CC/CV algorithm with proper termination:
• Pila IBC: use this to charge 1 or 2 batteries at 600mA
• 4sevens: single-bay charger, switchable between 500mA and 1000mA charging rates
• Cottonpickers: uses clips and magnets instead of a bay, and works off a USB port. You can order multiple configurations which have different charge rates. For example, I have a charger that I can set to 200mA or 500mA charge rates, to handle relatively bigger or smaller batteries (this is the only charger I'd use to charge a 10440, since I can charge that at a safe 200mA rate). This charger can also be bought with a built-in voltmeter so you can see the voltage on your batteries.
I specifically avoid and do not recommend any of the products from the *fire companies, etc., in my opinion they've been shown to have inconsistent quality. I'm not claiming the ones above are the only safe choices, but just ones I personally feel good about.
You also want to have a way to check the voltage on your batteries. An inexpensive Digital Multimeter (DMM) is generally good enough. The built-in voltmeter on a Cottonpickers charger is good as well. Thus armed, you can:
• Check the voltage of your batteries off the charger, to make sure they're not being dangerously overcharged
• Check the voltage of your batteries after use or long-term storage, to see if the voltage has dropped dangerously low (<1.5V), or if the protection circuit has tripped (0V).
• In multi-battery lights in which the batteries are in series, you can try to ensure you are using two batteries with about the same capacity, which will help avoid dangerous conditions that can occur when batteries are not balanced.


