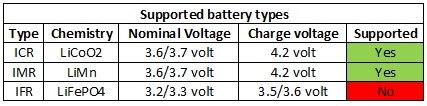
Re: Feedback requested: Li Ion beginner primer
Hi Joe. Your looking pretty good, I'd say. One thing that may be OK as it is, but most LiCo cells of 18650 size and larger have a maximum recommended charge rate of 0.7-0.8 C, or some even considerably less. You can probably get away with charging most of these cells at a 1C rate, but it's not good for them. You may want to work this point in somehow.
What exactly is supposed to happen when you try to charge a Li-Ion battery that has been over-drained?
Just to add something here. The biggest danger in over discharging cells is that at a voltage of around 1.5 Volts/cell, copper shunts can form within the cell, which as Joe mentioned, can cause the cell to short internally. These shunts may, or may not form, but the longer the cell is in a low voltage state, the more likely this may happen.
The scariest thing about these shunts is, while a short is probably more likely to occur when attempting to charge the cell, it could also happen at any time, while the device is in use, or even when not in use, such as in your pocket, or stored in a drawer. The other thing about these shunts is once they start to form, they continue to grow and the condition is irreversible. This results in a cell or battery that may seem fine one day, but may develop internal shorts the next day, after a week, or even months later. Of course, it's also possible that shunts never started to form, in which case, nothing will happen. The only way to tell for sure, is to disassemble the cell and look at it with an electron microscope.
With something like a cellphone, usually the built in protection circuitry will prevent the cell, or cells from being discharged to a low enough voltage to cause problems. If on the other hand, a battery is drained to the point where the protection circuitry has activated, and then the battery is not used for sometime, the voltage may drop lower. Then, there may be a problem.
It is important to remember, that the discharge graph of most any cell drops like a rock at a certain point. In the case of Li-Ion cells, this point is very close to the cutoff voltage of most protection circuits. So, while self discharge of Li-Ion chemistry cells is very low, it doesn't take much for the voltage to drop significantly, once you're headed over the cliff.
Dave